
The Objectives: |
For more details please refere to the following description. |
The Results: |
|
Participants: AU (AMS), AAU (JM and MLC); DTU (LMO); SDU (KR)
Description
Lake Ormstrup is located in an area with high levels of animal husbandry and requires arable land to dispose of N and P rich animal manures and slurries. The application of excessive amounts of manure and slurries to soil brings about the accumulation of nutrients in soils that can go on to pollute surface and groundwater (Szogi et al., 2015). In Denmark, both national P application limits and EU Nitrates Directive (91/676/EEC) limit application of sludge to land. Furthermore, these values vary depending on current P loading and soil type (Amery and Schoumans, 2014). When dredging Lake Ormstrup the P and nitrogen-rich sediment will be brought to the surface and this material requires treatment and disposal. When dredging the lake sediment, the dredging process overwhelmingly lifts pond water and not sludge giving a slurry consisting of 95 % water. Dewatering in WP2 will increase the solids content to above 15 % but if the P and nitrogen-rich sediment were to be transported to areas requiring P and nitrogen, this would be costly and carbon-intensive as you are paying to transport 85 % water. Drying sludge is energy-intensive, requiring 2.3 MJ of energy to raise 1 kg of water from 20ºC to 101ºC (g). To dry one ton of dewatered slurry 2000 MJ of energy is required to make just 150 kg of dried slurry. WP5 will develop an unorthodox hydrothermal dewatering and carbonization process based on an adapted hydrothermal carbonization (HTC) process to overcome this problem. HTC works using pressurized high temperature water (up to 250 ºC) to mimic natural coal formation, converting organic material within the sediment to a coal like material between one and 100 million times faster than nature (Titirici et al., 2007). The resulting biocoal, known as a hydrochar is hydrophobic (repels water) and is therefore easy to separate from the remaining water. In the proposed work, the dewatered lake sediment will be pumped into a heat exchanger raising the pressure to approximately 50 bar before being heated to 250 ºC. This pressure keeps the water liquid, avoiding latent heat of vaporization, therefore only requiring 0.9 MJ/kg water, with which 90 % of this energy should be recovered when the outgoing slurry heats the incoming slurry in the heat exchanger. Therefore treatment requires 77 MJ/ ton heat input as opposed to 2000 MJ/ ton for thermal drying.
Once hydrothermally dewatered and carbonized this research intends to transport the material for use as a soil improver, biofertilizer and commercial plant growth substrate. To the best of our knowledge HTC of lake sediments has not been attempted, therefore optimization of conditions and overcoming inhibition is required R&D for this project, along with the potential for biomass additions to improve the hydrochars physio-chemical properties.
In addition to the physical properties of the char, R&D will focus on the fate of P and nitrogen during HTC. To achieve this, the research will focus on two potential options (i) retaining P and nitrogen within the char for use as a biofertilizer (WP7) or (ii) extraction of P and nitrogen from the sediment and into the aqueous fraction for subsequent removal and recovery (WP 3). The former is theoretically technically simpler as P can be retained within the char by controlling the inorganic chemistry and process conditions (Ekpo et al., 2016; Smith and Ross 2016; Smith et al., 2016) and pot trials undertaken within WP7 will demonstrate how these parameters influence P availability to plants to enable product optimization. The latter route would require acid catalyzed HTC (brought about through recycling of the organic acid fraction or by adding additional cellulosic biomass) solubilizing P for recovery. The latter is regularly hypothesized and seldom demonstrated (Hielmann et al., 2014; Dai et al., 2015; Ekpo et al., 2016); however, work undertaken in WP3 provides a unique opportunity to overcome this.
To date, a largely unexplored processing option is the removal of heavy metals from sediment. As previously discussed in WP4 heavy metal modality varies between sediments, but desorption is often necessary. In EDR, this is achieved by lowering pH via water splitting. In HTC similar reductions in pH are seen as water goes subcritical (i.e when water goes above 100 ºC but remains liquid) as the dielectric properties of water change at elevated temperature leading to increases in both hydroxyl and hydronium ions. When organic polymers such as cellulose are present, these ions catalyze the hydrolysis of these polymers to monomers and then organic acids such as acetic, lactic, propionic, levulinic and formic acids dropping the pH to around 3 (depending on the materials buffering capacity). This drop in pH will mobilize heavy metals present, which will become ionic. The fate of these heavy meals is at present unclear; under low pH and high organic acid loadings (acetate) divalent cations appear to remain within the process water. Combining HTC with subsequent treatment of the hydrochar slurry via EDR (WP4) may bring about enhanced recovery over and beyond HTC or EDR alone, with the DC electric field aiding the migration of the heavy metals such as cadmium to the cathode for removal. Conversely, the applicant in Smith (2018) hypothesized that trivalent cations may play a role in hydrochar formation. In this instance, HTC may further immobilize heavy metals such as chromium in which case EDR before HTC would be a desirable strategy when minimizing heavy metal accumulation.
The final part of this R&D is the development of a pilot / demonstration scale reactor at Lake Ormstrup. An innovative part of this is the development of autothermal operation, which we hypothesize can be achieved by implementing simple but innovative heat exchangers and staged wet oxidation, which will provide the required energy by oxidizing volatile fatty acids in the process water and may simultaneously overcome phytotoxicity through exothermic degradation of the hydrochars phytotoxic compounds (Reza et al., 2016). The proposed reactor is given in Figure 6 but under the initial scoping of material behavior during HTC it will be determined whether high temperature solid and liquid fractionation is possible. If so, a further adaptation to the reactor design will enable catalytic wet oxidation of the process water increasing thermal output and providing cleaner process waters for P and nitrogen recovery, shown in Figure 7.
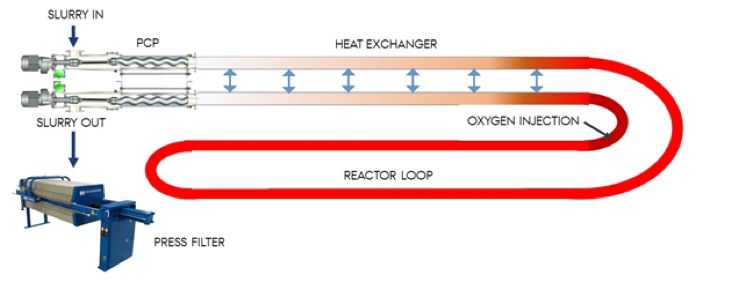
Figure 6 Proposed single phase hydrothermal reactor
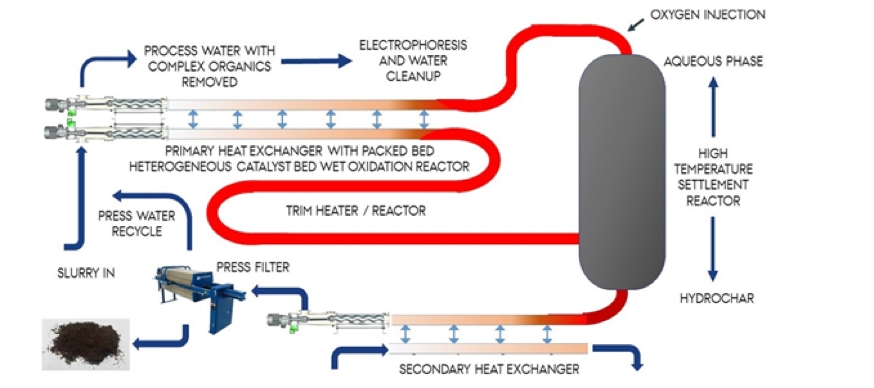
Figure 7 Proposed high temperature settlement reactor
Objectives:
- identify optimum conditions for HTC on lake sediment
- develop in reactor wet oxidation technology to both remove complex organics from the process water and supplement or even meet the heat requirements of the reaction
- incorporate the aforementioned objectives into a pilot scale autothermal hydrothermal reactor at the Ormstrup site.
- demonstrate hydrothermal dewatering at pilot scale
- demonstrate the dissolution of metals, phosphorus and nitrogen into the aqueous phase and their subsequent chemical recovery
Research tasks
Task 5.1. Establishing the influence of temperature and resonance time on the dewatering behavior of the hydrochar.
To date most HTC research is based on lignocellulosic biomass or sewage sludge. There appears very limited data on the behavior of lake sediment. Based primarily on observation, hydrothermal dewatering appears to be correlated to the extent to which the feedstock is dehydrated, which occurs primarily through removal of hydroxyl groups within the feedstock. Dehydration is quite closely related to reaction severity, a factor which combines temperature and pressure parameters into a single factor centered around the principle that similar products can be achieved through either increased retention times at a lower temperature or increased temperature with lower retention times. Therefore you can increase process severity by either increasing process temperature or increasing resonance time. Increasing both temperature and resonance time come with associated costs, principally in relation to reactor throughput and energy use. Furthermore, to date, there appears very limited data within the literature that quantifies how dewatering behavior is influenced by temperature, with literature almost solely focusing on yields and carbon density. This initial work will seek to address this by quantifying how reaction severity influences dewatering behavior using Kozeny-Carman theory while also identifying key reaction parameters required for pilot scale operation.
Task 5.2. Establishing the influence of temperature and resonance time on the behavior of phosphorus, nitrogen and metals, and the production of organic compounds present within the aqueous phase.
In addition to the dewatering behavior of the sludge, reaction severity will influence the mobility and retention of P, N, and metals present, with inorganics and heteroatoms likely to undergo both solution and reabsorption with increasing process severity. Coupled with this is the production of volatile fatty acids which will be present within the aqueous phase, with reaction severity influencing their yields. It is intended that wet oxidation of the process waters is utilized, which leads to the oxidative destruction of more complex VFA. However, it is likely that smaller VFA such as levulinic, acetic and formic acid will still be present and will be recovered alongside P at the cathode during electrophoresis. Given a primary objective of this work is to leach metals, P and nitrogen into the aqueous phase, this work will seek to identify optimum processing conditions to enable maximum inorganic and heteroatom recovery.
Task 5.3. Establishing the influence of pH on inorganic and heteroatom mobility.
It is documented that low pH increases mobility of P and metals into the aqueous phase. During HTC of lignocellulosic biomass pH of the process water will typically drop to 3 due to organic acids such as acetic, lactic, propionic, levulinic and formic acid produced in the initial hydrolysis and dehydration of cellulosic material. In lake sediment, this is less clear as the process chemistry is at present unknown and the material is likely to have greater buffering capacity than lignocellulosic material, albeit organic acids will still be present. To overcome this it is proposed that organic acids such as formic and acetic acid are recovered from the process water from the cathode on the electrodialysis unit and recycled to reduce pH. To understand the mechanisms small batch scale HTC experiments will be undertaken using formic acid as a model acid at different molarities / pH. Results will be compared to a mineral acid (e.g. hydrochloric acid) at different pH to see if there is a difference in process chemistry between organic and mineral acids present.
Task 5.4. Intelligent blending of biomass
In addition to recycling of concentrated organic acids incorporation of additional organic material in the form of low cost lignocellulosic biomass, will both increase organic acid production, lowering pH and provide additional carbon to the reaction, which should increase hydrothermal repolymerisation reactions, increasing char yield, further aiding post treatment dewatering and improve the agronomic properties of the hydrochar. Incorporation of this biomass will be either during the mechanical dewatering (WP2) where incorporation of biomass as a filter aid should aid dewatering on the vibrating membrane as presence of biomass should by increasing the permeability of the filter cake enhancing the dewatering efficiency. The biomass will subsequently then be present when it enters the HTC reactor. A second application point would be immediately before the HTC reactor and would be added to increase dry matter of the incoming material. This would enable higher recycling of the process waters that have been shown to increase metal and P recovery and catalyze the HTC process, enhancing the product quality or enabling reductions in the reactor resonance time (increases throughput) or reductions in temperature (increases thermal efficiency). This task will investigate the influence of different blending rates, processing temperature and water recycling to identify potential optimum processing conditions.
Task 5.5. Influence of wet oxidation on process water quality energy yield
The process water chemistry varies depending on reaction temperature, with sugars, furans, phenols and aldehydes present when processed at 200 ºC, with these compounds largely being degraded to organic acids or reincorporated into the char phase when processed at 250 ºC. By oxidizing HTC process waters the more complex phenols, aldehydes and furfurals are degraded to more simple organic acids, which are considered less problematic and therefore post HTC wet oxidation of process waters has been proposed as a method of water treatment for HTC process waters. This reaction is an exothermic reaction typically done at temperatures above 160 ºC (with greater efficiency associated with greater reaction temperatures), and is in theory autothermal, albeit this is dependent on the organic acid loading. What this research proposes is different. Rather than cool the reaction slurry, separate the process water, heat it back up and then oxidize the organics present; incorporate the wet oxidation process into the HTC process. The underlying hypothesis is that the char fraction, being stable aromatic carbon, will be resistant to oxidation and therefore if oxygen is present in the reactor slurry, it will largely oxidize the non-aromatic dissolved organics as opposed to the char phase. Therefore if oxygen is added to the reaction once the reaction comes to completion (i.e. when the slurry enters the heat exchanger to be cooled), wet oxidation will yield heat energy, raising the temperature within the heat exchanger and further increasing the heat within the incoming slurry, while oxidizing potentially problematic dissolved organics in the process. This task will investigate the calorimetry of the said reaction using a bespoke differential scanning reactor, assess the influence incorporation of wet oxidation has on process water and char chemistry and incorporate wet oxidation into the current AU continuous HTC system.
Task 5.6. testing and development of a high-temperature char settlement reactor
The slurry in the current continuous reactor design remains a single emulsion throughout the HTC process, therefore a dry matter of 15 % enters, and 100 % of that matter leaves via the outlet pumps and passes through the heat exchanger (see Figure 3). Different slurries behave differently at elevated temperatures; however, marked separation of the phases has been seen within the batch reactors for lake sediments, albeit using ‘as received’ sludge at low solids content. Settlement within the plug flow reactor tubes could be problematic during continued operation but may also offer an opportunity to improve thermal efficiency and scalability of the design. The proposed reactor is set out in Figure 7, would consist of a large settlement tank immediately after the heat exchangers and trim heater, and will retain the heated slurry at temperature for the desired resonance time. While residing at temperature it is envisioned that the sediment will separate with the solids removed at the bottom and liquid fraction at the top, albeit waters will be recirculated within the tank to ensure some fluidization. This reactor design has two advantages; (i) the sludge should naturally dewater, reducing post reaction water/solid separation and (ii) the liquid fraction can be passed though smaller diameter pipes on return though the wet oxidation stage and heat exchanger increasing heat recovery. Furthermore, removal of the solid phase will enable utilization of heterogeneous wet oxidation catalysts within the wet oxidation stage and heat exchanger increasing heat generation. To undertake this work, initial observations will be undertaken based on batch reactions and operation of the continuous reactor on dewatered sludge. Based on the observations an appropriately sized tank will be designed and fabricated and integrated into the existing AU continuous HTC reactor.
Task 5.7. Development of catalytic wet oxidation of process water
Commercial heterogeneous wet oxidation catalysts are available for treatment of aqueous chemicals and will result in greater oxidation of organics present, catalyze oxidation at lower temperatures and increase temperature output. Use of heterogeneous catalysts are not possible with integrated wet oxidation due to the presence of solid material within the slurry. Subject to successful demonstration of high-temperature settlement and separation, use of heterogeneous wet oxidation catalyst within the wet oxidation stage and heat exchanger will be possible, increasing the thermal efficiency of the process. To undertake literature review of potential heterogeneous catalysts will be undertaken, identifying potential candidates, which would ideally be commercially available. Testing will initially be undertaken using the bespoke differential scanning reactor used in task 5.5. It will assess the influence catalytic wet oxidation has on process water chemistry and thermal output and potential poisoning due to metals and P present in the process water. The best performing catalysts will be loaded into heat exchangers and tested in continuous operation at Ormstrup for extended periods of time.
Task 5.8. Operation of pilot scale HTC reactors at Lake Ormstrup
Due to development and construction costs, the AU Foulum HTC reactor will be housed in a shipping container and moved to Lake Ormstrup for an initial few months. During this period, the system will be tested in the ‘real world’ environment highlighting the need for modifications and optimal operating parameters (biomass feed, pH, water recycle ratio etc.). Following the initial field testing modification to the system will be undertaken incorporating optimized wet oxidation, settlement reactor stage, potential upgrades to heat exchangers and wet oxidation catalysts (if possible). The modified system will then be run for a second period at Lake Ormstrup testing performance of differing resonance times, different catalysts, loading and stability, etc. These subsequent trials will be regarded as main technology demonstration, with the first period considered a scoping run for the subsequent modifications.
Structure
AMS will be the leader of WP5. Two postdoc researchers will deliver the research in the WP for the first two years hosted and supervised at AU. Close collaboration will be maintained throughout the process with partners at SDU, DTU and AAU. It would be desirable to maintain an option to convert one postdoc researcher option to a PhD if an appropriate opportunity becomes available. In which case MLC (AAU) would be approached as co-supervisor with the thesis focusing on hydrothermal dewatering for sludge management.
Path to commercialization
A number of demonstration-scale HTC reactors are now in operation, all using different configurations, feedstocks and treatment temperatures. The applicant believes the proposed demo/ pilot plant proposed in this work is both scalable and cost-effective; therefore, positive development and demonstration of this work should translate to a commercial solution. Furthermore, demonstration of wet air oxidation and proposed heat exchanger systems should mean limited energy input, which will be game changing for lake restoration alongside WWTPs and livestock farming. AU is in close collaboration with Circlia Nordic/ bio2oil who provide commercial scale hydrothermal plants and could amongst others be a potential technology provider to take the findings of this research to market. “Ready for market” HTC holds potential applications in several of Dansk Ingeniør Service business areas, so they may also have commercial interests in the technologies application in a number of different fields and may also provide a route to commercialization.
Risks associated with work package
Risk 1: Appropriate infrastructure and obtaining appropriate permits not possible at Lake Ormstrup.
The hydrothermal equipment is currently located at Aarhus University Foulum 25 km away. Equipment can remain at Foulum and sediment transported to Foulum for processing via tanker by road.
Risk 2: Process water too high in organics to be treated on-site.
Operation on-site without water treatment technologies developed in WP 3 would require the discharge of water into mains sewer or transport process water off-site for water treatment.
